Pharmaceutical transportation management is crucial to the healthcare industry. Pharmaceuticals, including drugs and vaccines, represent a significant portion of global trade, necessitating a specialized focus on their safe transportation. The failure to execute a safe and timely delivery not only disrupts the medical supply chain but can also compromise the efficacy of these critical products, potentially endangering lives.
Stockouts or delays in pharmaceutical delivery can wreak havoc on healthcare services, escalating treatment costs and potentially causing adverse health outcomes for patients. Additionally, non-compliance with safety and regulatory guidelines can result in costly fines, damaged brand reputation, and loss of customer trust. Therefore, ensuring safe transport is not just an operational imperative but a critical aspect of patient care and safety.
Understanding the Specifics of Pharmaceutical Transport
The transport of pharmaceutical products differs significantly from standard parcel shipping due to the sensitive nature of these goods. Many pharmaceutical products require stringent temperature controls, while others may be susceptible to damage from light, humidity, or vibration. Such products often demand specialized containers, handling procedures, and storage conditions.
Compared to other goods, pharmaceuticals present a unique array of challenges during transit, among them are temperature excursions, damaging light exposure, humidity variations, and turbulence. It is, therefore, mandatory to extend full attention to every aspect of the pharmaceutical transport process, ensuring the safe and dependable delivery of these life-saving supplies.
Top Considerations for Safe Pharmaceutical Transport
To maintain the effectiveness and quality of pharmaceutical products during transit, it’s crucial to control several environmental conditions. Among them, temperature control takes precedence, with many drugs requiring storage under strict ‘cold chain’ conditions. Pharmaceutical transport services must, therefore, offer reliable temperature-controlled logistics equipped with both refrigerated and ambient storage capabilities.
Apart from temperature, correct humidity levels and limited light exposure are vital to maintaining drug potency and stability. High moisture levels can cause mould growth or reactions with drug substances, while excess light may degrade certain drugs, reducing their efficacy. Proper management of these parameters is, thus, an indispensable part of pharmaceutical transport.
Investing in High-Quality Packaging
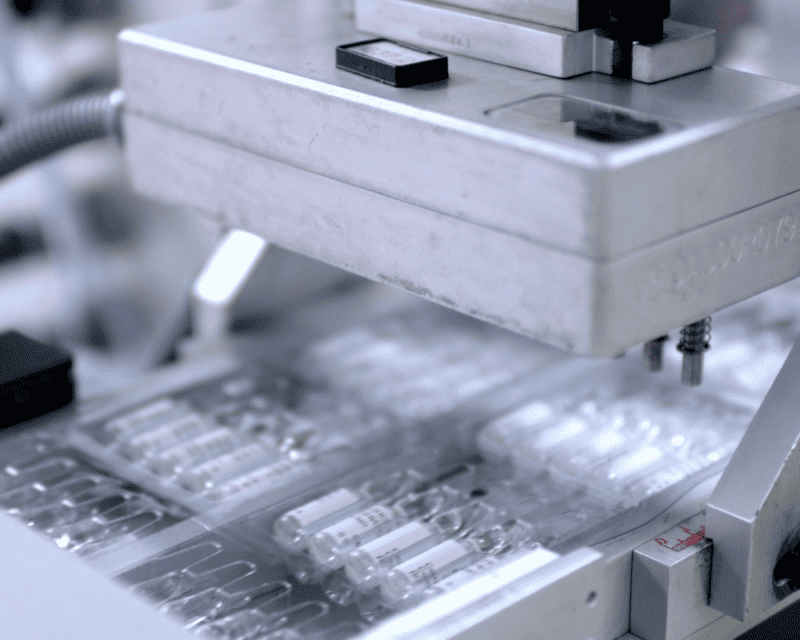
High-quality packaging plays a pivotal role in pharmaceutical transport. Secure packaging prevents contamination, provides protection from light, maintains an optimum environment, and safeguards the products from physical damage during transport. Pharmaceutical packaging, therefore, must meet high standards of integrity and security to ensure that pharma products reach consumers in perfect condition.
Inadequate packaging can lead to undue exposure to environmental hazards such as temperature changes, light, or moisture. It can also precipitate physical damage during handling and transport. Thus, optimal pharmaceutical packaging should offer thermal insulation, protection against humidity and light, shock absorption, and tamper-proof features.
Maintaining Proper Temperature Control
Maintaining consistent temperature controls during the transport of pharmaceutical products is of paramount importance. Temperature fluctuations can degrade drugs, reduce their potency, and compromise their safety. For instance, vaccines and biologics can lose their effectiveness if exposed to sub-optimal temperatures, leading to a significant public health risk.
Most pharmaceutical products require stringent temperature ranges, often necessitating refrigerated transport. However, each product may have different temperature requirements, making it crucial to have a logistics provider capable of providing customized temperature-controlled solutions. Transport companies must use validated temperature-controlled containers and monitor temperature in real-time to ensure pharmaceutical products remain within their optimal temperature range throughout transit.
Importance of Humidity Control
Controlling humidity levels during pharmaceutical transport can significantly impact the integrity and stability of products. High humidity can cause moisture damage, including mould growth or antibiotic degradation. Conversely, low humidity can lead to evaporation, weight loss, and structural changes in pharmaceuticals.
Given the above, strategies to control humidity levels during transport are crucial. These include using moisture barrier packaging, desiccants, and humidity-controlled logistics units. It’s also essential to monitor moisture levels in real-time, taking corrective action in case of any deviations from stipulated ranges.
Regulations governing pharmaceutical transport are complex and varied, spanning across different jurisdictions worldwide. These regulations cover a variety of critical areas like temperature management, labelling, documentation, and tracking, to name a few. It’s crucial that pharmaceutical transporters, therefore, have a thorough understanding of these regulations to ensure full compliance.
Failure to comply with these regulations can lead to severe consequences, ranging from hefty fines to the withdrawal of operating licenses, product recalls, or even criminal charges. Hence, it’s vital for transport firms to be proactive in their regulatory compliance, investing in appropriate systems, resources, and personnel training to avoid any potential breaches.
Harnessing Technology for Improved Transport Safety

Advancements in technology offer unprecedented opportunities to enhance pharmaceutical transport safety. Technologies like GPS tracking can enable real-time location monitoring, providing valuable insights into any potential delays or unforeseen challenges.
Similarly, Internet of Things (IoT) devices like temperature and humidity sensors can offer real-time data on environmental conditions in transit, alerting logistics managers to any deviations from approved ranges. Other technology solutions, like AI-based predictive analytics, can help forecast demand and optimize schedules to prevent stock-outs and ensure timely delivery.
Having a Contingency Plan
In the unpredictable world of transport and logistics, having a contingency plan is crucial. Despite best efforts, things may not always go as planned. There may be unforeseen delays due to weather, equipment breakdowns, or operational failures. A well-drafted contingency plan can offer valuable guidance in such situations.
The contingency plan should offer direction on alternate transport arrangements, additional resources, emergency contacts, and steps for prompt communication with all stakeholders. Having this information readily available can help minimize disruption and ensure swift recovery in the face of unforeseen challenges.
Staff Training and Regular Audits
Staff training is an often overlooked but critical aspect of ensuring safe pharmaceutical transport. Well-trained staff can understand and execute standard operating procedures, adhere to safety guidelines, and respond promptly to any contingencies. They are the first line of defense in maintaining the integrity and safety of pharmaceuticals during transit.
Additionally, regular audits of logistics operations can help identify potential gaps or risks in the transport process, enabling proactive correction and prevention of potential mistakes. Audits also demonstrate due diligence in regulatory compliance, reducing the risk of non-compliance penalties.
Conclusion
In conclusion, transport is an essential link in the pharmaceutical supply chain, requiring focused attention on safety, quality, and compliance. By understanding pharmaceutical transport’s unique challenges, we can better adapt protocols to ensure the safe delivery of critical healthcare supplies.
From managing environmental conditions, investing in high-quality packaging, navigating complex regulatory landscapes, staff development, and harnessing technology, these measures collectively guarantee that pharmaceutical products are securely delivered, conserving their potency and effectiveness just as when they left the manufacturer.
Remember, safety and security are your top priorities when transporting pharmaceutical goods. It’s not just about speed and efficiency but about maintaining the integrity of the goods, complying with regulations and ultimately ensuring patient safety and satisfaction. It’s a huge responsibility, but with the right procedures and due diligence, it’s one you can successfully carry out.
